MRSA Staph Infection
History
Methicillin-resistant Staphylococcus aureus (MRSA) is a bacterium that causes infections in different parts of the body. It's tougher to treat than most strains of staphylococcus aureus -- or staph -- because it's resistant to some commonly used antibiotics.
The symptoms of MRSA depend on where you're infected. Most often, it causes mild infections on the skin, causing pimples or boils. But it can also cause more serious skin infections or infect surgical wounds, the bloodstream, the lungs, or the urinary tract.
MRSA - Unsanitary Shaving Razors
Skin-to-skin contact, cuts or abrasions of the skin, contaminated items and surfaces, crowded living conditions and poor hygiene have all been associated with the transmission of MRSA in the community. The smallest break in the skin - even razor burn - can allow bacteria to enter the body and cause infection.
Staph infections, including MRSA, generally start as small red bumps that resemble pimples, boils or spider bites. These can quickly turn into deep, painful boils or abscesses that require surgical drainage. The wound drainage and pus is very infectious. Good hygiene is the most effective way to prevent MRSA infections and to prevent the recurrence of treated wounds.
Keeping Up Personal Hygiene
“My daughter’s started with a slight razor burn in her underarm. Within a few days a large boil existed under her skin and it was extremely painful. We took her to the Doctor’s office and they drained it and sent in a sample of what was extracted for lab work. The sample came back as MRSA and that is when I started to do my research.”
Community Associated Resistant Staph
Use clean razors. Some people habitually share razors or combs. This is a no-no. Since a person can unknowingly carry the bacteria that causes staph infection, using another person's comb, brush, towel or razor can increase your risk of infection. Plus, razor cuts are common. It only takes a small nick or cut to transmit the bacteria.
Prevent Staph Infections
With Razor Gator™, the growth of bacteria on razors is reduced. In essence, Razor Gator™ can reduce the chances of getting MRSA from even the slightest razor burns or cuts.
See the Razor Gator Research. |
|
Clinical Concerns:
Garden-variety staph are common bacteria that can live on our bodies. Plenty of healthy people carry staph without being infected by it. In fact, 25-30% of us have staph bacteria in our noses. But staph can be a problem if it manages to get into the body, often through a cut. Once there, it can cause an infection. Staph is one of the most common causes of skin infections in the U.S. Usually, these are minor and don't need special treatment. Less often, staph can cause serious problems like infected wounds or pneumonia.
Staph can usually be treated with antibiotics. But over the decades, some strains of staph -- like MRSA -- have become resistant to antibiotics that once destroyed it. MRSA was first discovered in 1961. It's now immune to methicillin, amoxicillin, penicillin, oxacillin, and many other antibiotics. While some antibiotics still work, MRSA is constantly adapting. Researchers developing new antibiotics are having a tough time keeping up.
Understanding Staph
image-2, MRSA infection on leg,
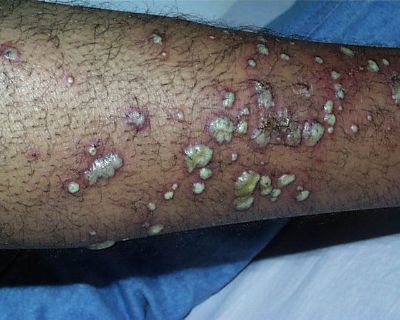
Staph On Leg - Click for larger image
Symptoms
Most MRSA infections are skin infections that produce the following signs and symptoms:
Cellulitis (infection of the skin or the fat and tissues that lie immediately beneath the skin, usually starting as small red bumps in the skin)
Boils (pus-filled infections of hair follicles),
Abscesses (collections of pus in under the skin),
Sty (infection of eyelid gland),
Carbuncles (infections larger than an abscess, usually with several openings to the skin),
Impetigo (a skin infection with pus-filled blisters).
Staph Article
Image-3, Cellulitis on face,
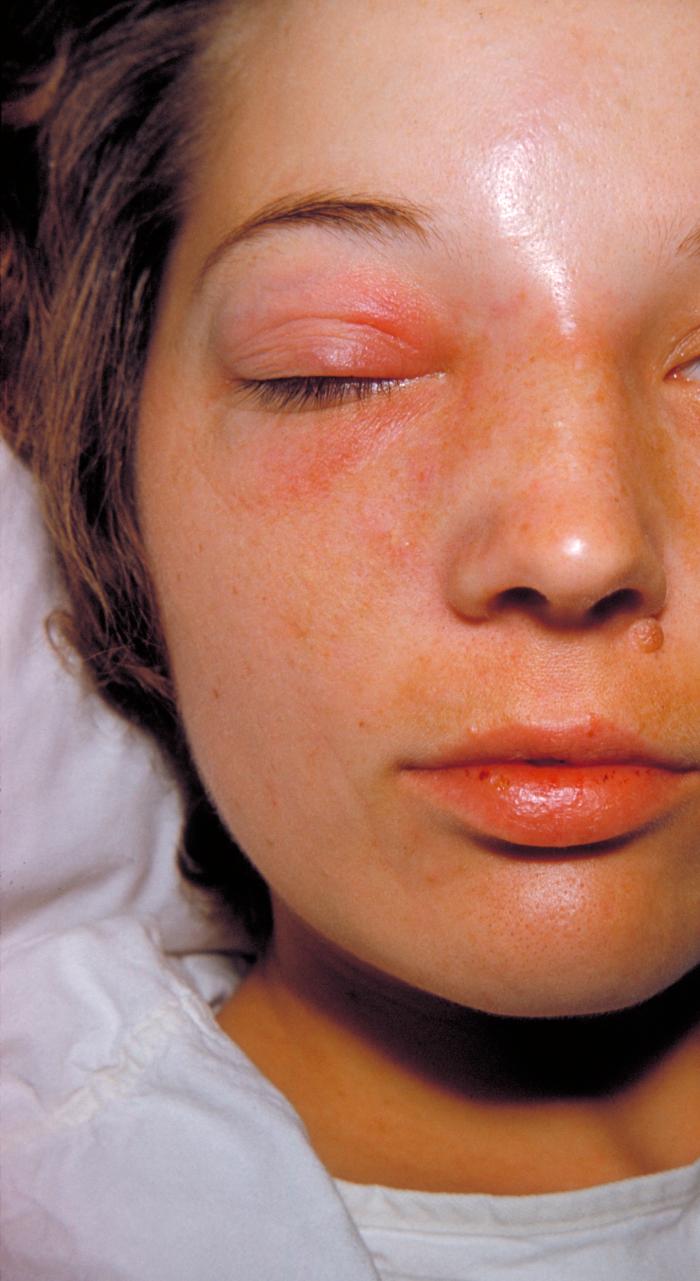
Cellulitis - Click for larger image
Image-4, Boil on neck,
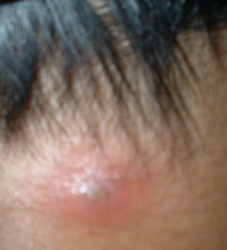
Boil - Click for larger image
Image-5, abscess,
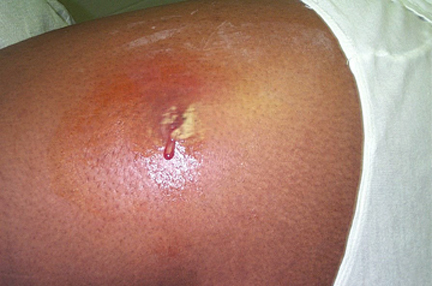
Abscess - Click for larger image
Image-6, sty,
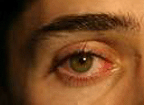
Sty - Click for larger image
Image-7, carbuncles,
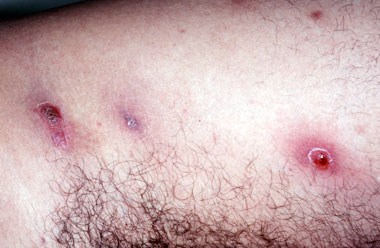
Carbuncles - Click for larger image
Image-8, impetigo on baby,
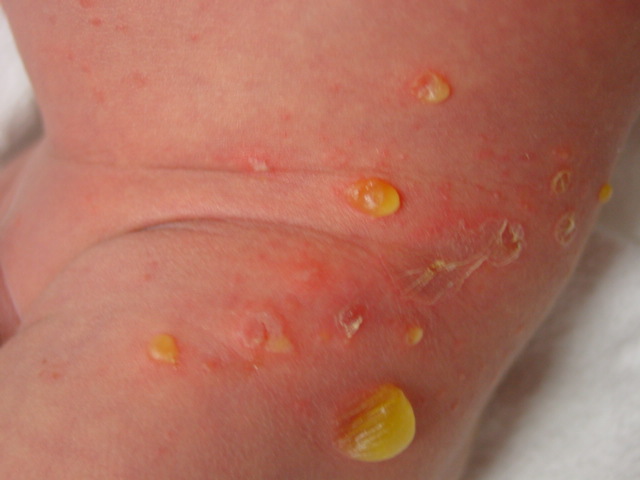
Impetigo On Baby - Click for larger image
Prevention
Staph is spread by contact. You can get MRSA if you touch a person who carries the bacteria -- or if you touch something that an infected person touched.
The CDC says that the following things have been associated with the spread of MRSA.
Close skin-to-skin contact
Openings in the skin, like cuts or abrasions
Contaminated items and surfaces
Crowded living conditions, like in hospitals or prisons
Poor hygiene
In health care centers, people who carry MRSA are sometimes isolated from other patients to prevent the bacteria from spreading. According to the CDC, here are some of the best ways to prevent MRSA.
Wash your hands. Use soap and water or an alcohol-base hand sanitizer. Also, wash thoroughly. Experts suggest that you wash your hands for as long as it takes you to recite the alphabet.
Image-9, good hygiene,

Good Hygiene - Click for larger image
Cover cuts and scrapes with a clean bandage. This will help the wound heal. It will also prevent you from spreading bacteria to other people.
Image-10, clean bandage,
Clean Bandage - Click for larger image
Do not touch other people's wounds or bandages.
Do not share personal items like towels or razors. If you use any shared gym equipment, wipe it down before and after you use it. Drying clothes, sheets, and towels in a dryer -- rather than letting them air dry -- helps kill bacteria.
MRSA Prevention
Because hospital and community strains of MRSA generally occur in different settings, the risk factors for the two strains differ. Risk factors for hospital-acquired (HA) MRSA include:
A current or recent hospitalization. MRSA remains a concern in hospitals, where it can attack those most vulnerable ??? older adults and people with weakened immune systems, burns, surgical wounds or serious underlying health problems. A 2007 report from the Association for Professionals in Infection Control and Epidemiology estimates that 1.2 million hospital patients are infected with MRSA each year in the United States. They also estimate another 423,000 are colonized with it.
Image-11, hospital bed,

Hospital Bed
Residing in a long-term care facility. MRSA is far more prevalent in these facilities than it is in hospitals. Carriers of MRSA have the ability to spread it, even if they're not sick themselves.
Image-12, long-term care facility-inside,

Long Term Care Facility - Click for larger image
Invasive devices. People who are on dialysis, are catheterized, or have feeding tubes or other invasive devices are at higher risk.
image-13, dialysis machine,
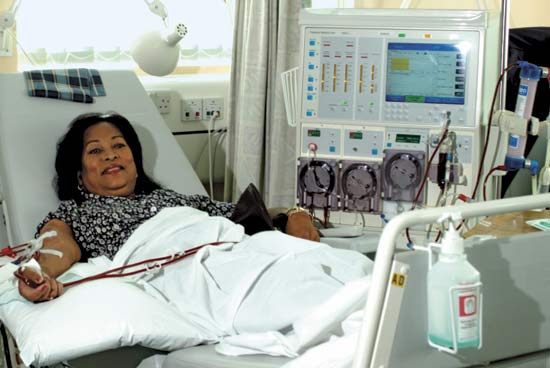
Dialysis Machine - Click for larger image
Recent antibiotic use. Treatment with fluoroquinolones (ciprofloxacin, ofloxacin or levofloxacin) or cephalosporin antibiotics can increase the risk of HA-MRSA.
image-14, ciprofloxacin,

Ciprofloxacin - Click for larger image
These are the main risk factors for community-acquired (CA) MRSA:
Young age. CA-MRSA can be particularly dangerous in children. Often entering the body through a cut or scrape, MRSA can quickly cause a wide spread infection. Children may be susceptible because their immune systems aren't fully developed or they don't yet have antibodies to common germs. Children and young adults are also much more likely to develop dangerous forms of pneumonia than older people are.
Image-15, little kid drawing,

Little Kid Drawing - Click for larger image
Participating in contact sports. CA-MRSA has crept into both amateur and professional sports teams. The bacteria spread easily through cuts and abrasions and skin-to-skin contact.
Image-16, football,

Football - Click for larger image
Sharing towels or athletic equipment. Although few outbreaks have been reported in public gyms, CA-MRSA has spread among athletes sharing razors, towels, uniforms or equipment.
Image-17, don’t share towels poster,

Towels - Click for larger image
Having a weakened immune system. People with weakened immune systems, including those living with HIV/AIDS, are more likely to have severe CA-MRSA infections.
Image-18, African aids victim,

Aids Victim - Click for larger image
Living in crowded or unsanitary conditions. Outbreaks of CA-MRSA have occurred in military training camps and in American and European prisons.
Image-19, crowded prison,

Crowded Prison - Click for larger image
Association with health care workers. People who are in close contact with health care workers are at increased risk of serious staph infections.
Image-20, nurse,

Nurse - Click for larger image
DS00735/DSECTION=4
Epidemiology
The Changing Epidemiology of Staphylococcus aureus?
Henry F. Chambers
University of California San Francisco and San Francisco General Hospital, San Francisco, California, USA
--------------------------------------------------------------------------------
Strains of methicillin-resistant Staphylococcus aureus (MRSA), which had been largely confined to hospitals and long-term care facilities, are emerging in the community. The changing epidemiology of MRSA bears striking similarity to the emergence of penicillinase-mediated resistance in S. aureus decades ago. Even though the origin (hospital or the community) of the emerging MRSA strains is not known, the prevalence of these strains in the community seems likely to increase substantially.
Recent reports of strains of methicillin-resistant Staphylococcus aureus (MRSA) isolated from children in the community have led to speculation that the epidemiology of S. aureus is changing (1-3). Epidemiologic features of the cases described in these reports show a major departure from features typically associated with MRSA colonization or infection. Traditionally, MRSA infections have been acquired almost exclusively in hospitals, long-term care facilities, or similar institutional settings (4). Risk factors for MRSA colonization or infection in the hospital include prior antibiotic exposure, admission to an intensive care unit, surgery, and exposure to an MRSA-colonized patient (4,5).
Humans are a natural reservoir for S. aureus, and asymptomatic colonization is far more common than infection. Colonization of the nasopharynx, perineum, or skin, particularly if the cutaneous barrier has been disrupted or damaged, may occur shortly after birth and may recur anytime thereafter (6). Family members of a colonized infant may also become colonized. Transmission occurs by direct contact to a colonized carrier. Carriage rates are 25% to 50%; higher rates than in the general population are observed in injection drug users, persons with insulin-dependent diabetes, patients with dermatologic conditions, patients with long-term indwelling intravascular catheters, and health-care workers (7). Young children tend to have higher colonization rates, probably because of their frequent contact with respiratory secretions (8,9). Colonization may be transient or persistent and can last for years (10).
When cases of MRSA infection have been identified in the community, a thorough investigation usually reveals a history of recent hospitalization; close contact with a person who has been hospitalized; or other risk factors, such as previous antimicrobial-drug therapy (11,12). In the 1980-1981 outbreak of community-acquired MRSA infections in Detroit (13,14), approximately two thirds of the patients affected were injection drug users. Previous antimicrobial therapy was associated with infection by a strain of MRSA. Recent hospitalization, defined as within 4 months (which may not have been long enough, given that hospital-acquired MRSA colonization may last years [10]), was not a predictor of MRSA infection in the drug users; however, the epidemic strain had the same phage type as a strain of MRSA responsible for an outbreak in a burn unit in Minnesota in 1976 (15). The source of the Detroit outbreak was not identified. Frequent needle sharing was speculated to be the mode of transmission in the community. In contrast to infection in injection drug users, MRSA infection in nonusers was strongly associated with recent hospitalization, which suggests that drug users had become colonized during a previous hospital admission. In turn, patients (and probably health-care workers, who become colonized with MRSA as a consequence of their exposure to colonized patients) in a hospital or other health-care setting can then transmit MRSA strains to close associates and family members by direct contact.
Direct or indirect exposure to an institutional health-care setting in which MRSA is likely to be found and other risk factors typically associated with MRSA colonization are strikingly absent from the recently described cases in which MRSA seems to have been acquired from a community reservoir. The antimicrobial susceptibility patterns observed for these MRSA strains are further evidence of a possible community origin. Unlike hospital strains, which typically are resistant to multiple antibiotics and can be shown by typing schemes to be related to other hospital strains, these so-called community strains have tended to be susceptible to other antibiotic classes and often are resistant only to beta-lactam antibiotics (1,2,9). The lack or loss of resistance to multiple antibiotics suggests a community origin because antibiotic selective pressure is much lower within the community than in hospitals, and the survival advantage of multiple-drug resistance is lower. Typing by pulsed-field gel electrophoresis (PFGE) also suggests that these strains are distinctive.
Emergence of Penicillinase-Producing S. aureus
Whether their appearance in the community and their susceptibility to antibiotics other than beta-lactams are fundamental changes in MRSA epidemiology is debatable. The epidemiology of MRSA and the factors driving resistance bear strong similarities and parallels to those occurring with penicillin-resistant strains of S. aureus in the 1940s and 1950s. When Kirby's first description of penicillinase-producing strains of S. aureus was published in 1944 (16), resistance was infrequently encountered, with only a handful of strains available for study. As with MRSA, penicillinase-producing strains first were isolated from hospitalized patients (17). Community strains tended to be penicillin susceptible. The prevalence of penicillinase-producing strains of S. aureus within hospitals soon began to rise as penicillin became readily available after World War II. Within a few years, most hospital isolates were resistant to penicillin (17). As was observed decades later with MRSA, previous treatment with a beta-lactam antibiotic, in this case penicillin, increased the chances of isolating a penicillin-resistant strain. Colonization of hospital staff by penicillin-resistant strains and their role in transmission also were notable features of these early reports.
Although penicillinase-producing strains were universally present in hospitals by the early 1950s, community isolates of S. aureus were considered to be largely penicillin susceptible. Penicillin continued to be recommended as an effective anti-staphylococcal agent as late as the early 1970s (18). However, then as now, there was no systematic surveillance for antibiotic resistance among S. aureus isolates circulating within communities. The first comprehensive description and accurate assessment of the epidemiology of drug-resistant strains of S. aureus were published in 1969 by Jessen et al. (19). Examination of more than 2,000 blood culture isolates of S. aureus received at the Statens Seruminstitut in Copenhagen for 1957 to 1966 for which detailed information on the origin of infection (hospital or community) was available confirmed a high prevalence of penicillin resistance (85% to 90%) for hospital isolates of S. aureus. Somewhat unexpected was that penicillinase-producing strains were almost as common in the community, with 65% to 70% of isolates resistant to penicillin. The community-acquired isolates often were resistant only to penicillin, whereas nosocomial strains typically were resistant to multiple antibiotics.
By the 1970s, it was apparent that the high prevalence of penicillin resistance among community isolates was not limited to Denmark. A remarkably constant 70% to 85% prevalence of penicillinase-producing strains was found regardless of location in inner cities, suburbs, rural areas, within and outside the United States (8,20,21). A population-based study conducted in 1972 revealed that 47% of healthy school-aged children under 10 years of age were carriers of S. aureus and that 68% of colonizing strains were penicillin-resistant (8).
Table 1. Time required for prevalence rates of resistance to reach 25% in hospitals
--------------------------------------------------------------------------------
Drug Year drug introduced Years to report of resistance Years until 25% rate in hospitals Years until 25% rate in community
--------------------------------------------------------------------------------
Penicillin 1941 1-2 6 15-20
Vancomycin 1956 40 ? ?
Methicillin 1961 <1 25-30 40-50 (projected)
--------------------------------------------------------------------------------
Staphylococcal resistance was reported shortly after penicillin was introduced, and within approximately 6 years, 25% of hospital strains were resistant (Table 1). One to two decades later, 25% of community isolates were penicillin resistant (22, 23). Although the rates are only approximate because they are based on reports from numerous locations, a clear correlation exists between the prevalence of penicillin-resistant strains of S. aureus reported in hospitals and rates in the community (Figure). The upswing in community rates followed soon after nosocomial rates exceeded 40% to 50%, and by the 1970s, the two rates were practically equal.
Community-Acquired MRSA
In the past two decades, the prevalence of MRSA strains has steadily increased in hospitals in the United States and abroad. National Nosocomial Infections Surveillance (NNIS) data collected by the Centers for Disease Control in the early to mid-1980s indicated that MRSA were limited mainly to relatively large urban medical centers and that rates were 5% to 10%. Smaller, nonreferral centers were relatively free of MRSA, with prevalence rates well below 5%. By the 1990s, rates among these smaller (<200-bed) community hospitals had increased to 20%, and twice that rate was found in the larger urban centers. More recent surveillance data from NNIS indicate that rates have continued to rise, with the prevalence of MRSA isolates from intensive care units approaching 50% by the end of 1998. Unless this upward trend has reversed, the prevalence rate of MRSA in U.S. hospitals likely has reached 50%. At these high rates, the emergence of correspondingly high rates of MRSA strains in the community can be anticipated. Because no systematic, population-based surveillance of community isolates of S. aureus exists, the true prevalence of MRSA cannot be determined. One hospital-based study found that up to 40% of MRSA infections in adults were acquired before admission to the hospital (24). Published reports of MRSA colonization and infection among study participants who lack traditional risk factors indicate that community prevalence rates are rising. For the period 1976 through 1990, a Medline search identified 10 articles in which key words "methicillin-resistant Staphylococcus aureus" and "community" appeared in the title (Table 2). For the period 1991 through 1999, 39 articles were identified; 21 were published from 1996 through 1999. A community-based survey of injection drug users in the San Francisco Bay area communities found that up to 35% of S. aureus carriers harbored MRSA (Table 3).
Table 2. Estimated prevalence of methicillin-resistant Staphylococcus aureus strains in U.S. hospitals and publicationsa pertaining to community-acquired methicillin-resistant S. aureus
--------------------------------------------------------------------------------
Hospital prevalence Total no. No. of articles No. of articles
Years rate (%) of articles pertaining to children pertaining to other groups
--------------------------------------------------------------------------------
1996-1999 40 29 8 3 (seniors, rugby team, wrestlers)
1991-1995 28 10 0 0
1986-1990 20 5 1 0
1981-1985 5 5 0 4 (addicts)
1976-1980 <5 0 0 0
--------------------------------------------------------------------------------
aIdentified by Medline search.
Table 3. Outpatient population-based prevalence of Staphylococcus aureus carriage and percentage of carriers with methicillin-resistant (MRSA) strains among injection drug users
--------------------------------------------------------------------------------
Location S. aureus carriage (%) Carriers with MRSA (%)
--------------------------------------------------------------------------------
San Francisco
Western addition 25 16
Tenderloin 20 21
Mission 34 35
Bayview 23 12
East Bay
Oakland 18 12
Richmond 20 6
--------------------------------------------------------------------------------
In early reports, community isolates of MRSA had affected persons with known risk factors for colonization (contact with health-care facilities, previous antimicrobial therapy), whereas more recent reports describe colonization and transmission in populations lacking risk factors. A recent study of methicillin-resistant S. aureus carriage in children attending day-care centers is reminiscent of Ross's survey of healthy children colonized with penicillin-resistant S. aureus strains two decades earlier (9). This survey of two day-care centers in Dallas, Texas, each of which had an index case of MRSA infection, revealed that 3% and 24% of children in the respective centers were colonized. The isolates generally were susceptible to multiple antibiotics, which is in contrast to the typical, multiple-drug-resistant hospital isolate. Forty percent of the children colonized had had no contact with a health-care facility or a household member with such contact within the previous 2 years, which suggests that sustained transmission and colonization of MRSA in children were occurring in the community. A study from Chicago found a 25-fold increase in the number of children admitted to the hospital with an MRSA infection who lacked an identifiable risk factor for prior colonization (1). These MRSA strains, also presumably transmitted and acquired in a community setting, tended to be susceptible to multiple antibiotics. Two examined strains had PFGE patterns that were distinct from the common nosocomial isolates.
The deaths of four children from rural Minnesota and North Dakota caused by infection with community-acquired MRSA strains brought the problem to national attention in 1999 (2). These children, like those in the Chicago study, lacked risk factors for MRSA infection. The infections were caused by strains susceptible to several antibiotics, except beta-lactams. The PFGE patterns of these strains indicated that they were related to one another but differed from typical nosocomial isolates circulating in local hospitals.
These reports of infection and colonization by strains of MRSA in children provide compelling evidence that MRSA strains, like penicillinase-producing strains almost 30 years ago, have gained a foothold in the community and are emerging as important outpatient pathogens. Based on the experience with penicillin-resistant strains, prevalence of MRSA among community isolates may be as high as 25% within the next 5 to 10 years (Table 1).
Origins of Community-Acquired MRSA
The origins of these community-acquired strains are subject to debate. One possibility is that they are feral descendants of hospital isolates. If so, these isolates must have undergone considerable change because they possess distinctive PFGE patterns and have lost resistance to multiple antibiotics. Another possibility is that the community isolates arose as a consequence of horizontal transfer of the methicillin-resistance determinant into a formerly susceptible background. This possibility could also account for the unique PFGE patterns and lack of resistance to multiple drugs. In the case of penicillinase-mediated resistance, dissemination of strains from the hospital and horizontal transfer of the penicillinase gene into susceptible recipient strains were both likely to have contributed to emergence of penicillin-resistant strains in the community. Penicillinase typically is plasmid encoded and can be readily transferred by transduction or conjugation. These characteristics account for methicillin-susceptible, penicillinase-producing strains being genetically diverse and polyclonal.
Unlike plasmid-encoded penicillinase, the methicillin resistance determinant, mec, is chromosomally encoded. Horizontal transfer of mec is thought to be relatively rare; only a handful of ancestral strains account for all clinical isolates worldwide (25). Ribotyping (a genotyping scheme that uses Southern blot analysis to identify DNA restriction enzyme polymorphisms of the five to six ribosomal RNA genes distributed throughout the S. aureus chromosome) and cluster analysis indicate that mec has integrated into at least three distinct methicillin-susceptible chromosomal backgrounds, A, B, and C (26,27). mec itself is polymorphic; three types have been identified: I, II, and III. These polymorphs differ in number of base pairs, genetic organization, number of insertion sequences, and resistance determinants (Table 4). All three mec types have been found integrated into ribotype cluster A. Type II mec has also integrated into cluster B and C ribotype backgrounds. Thus, five distinct clones of MRSA have been identified worldwide since the first strain was isolated in the United Kingdom in 1961; even if more clones were identified, the relatively low number pales in comparison to the large number of distinct clones of methicillin-susceptible clones.
Table 4. Elements found within three types of mec-associated DNA
--------------------------------------------------------------------------------
mec types
--------------------------------------------------------------------------------
Genetic featurea I II III
--------------------------------------------------------------------------------
Size 32 kb 52 kb 60 kb
mecA + + +
mecR1-mecI - + +
ccrAB + + +
pUB110 - + -
IS431 (number) 1 2 4
Tn554 (number) 0 1 2
Tc, Hg resistance - - +
--------------------------------------------------------------------------------
amecA = gene encoding PBP 2a, the penicillin-binding protein with low binding affinity that mediates methicillin resistance; mecR1-mecI = sensor-transducer and repressor genes that regulate production of inducible PBP 2a; ccrAB = cassette chromosome recombinases A and B that mobilize the mec element; pUB110 = integrated plasmid that encodes tobramycin and kanamycin resistance; IS431 = insertion sequence; Tn554 = erythromycin-resistance encoding transposon; Tc = tetracycline-resistance determinant; Hg = mercury-resistance determinant.
Unlike the mechanisms responsible for horizontal transfer of penicillinase resistance, the mechanism by which mec might be mobilized and transferred had not been understood until recently. Hiramatsu and co-workers have identified two genes, ccrAB (cassette chromosome recombinase genes A and B), which are homologous to DNA recombinases of the invertase-resolvase family and can mobilize mec (28). The proteins encoded by these genes catalyze precise excision and precise site-specific and orientation-specific integration of mec into the S. aureus chromosome. Thus, mec is somewhat analogous to the pathogenicity islands found in gram-negative bacilli, except that this locus encodes resistance determinants instead of virulence factors. How an element as large as mec is transferred from donor to recipient is not known. Nevertheless, as the prevalence of MRSA strains has increased, so has the abundance of mec DNA. Even though transfer of mec occurs rarely, the chances that it might occur have correspondingly increased. The community-acquired strains could possibly have arisen as a consequence of one of these rare transfers of mec from a nosocomial donor into a susceptible recipient. With appropriate analysis of mec DNA and the recipient chromosome, researchers should be able to determine whether these newly identified community-acquired strains are feral or freestanding. Regardless of the origins, which are likely to become obscured as clones move back and forth between hospital and community over time, emergence of MRSA within the community is a major threat with several important clinical implications: treatment failure with accompanying complications or death may result if an antistaphylococcal beta-lactam antibiotic is used and the infecting strain proves to be resistant; infections caused by methicillin-resistant strains may be more difficult to manage or more expensive to treat, perhaps because vancomycin is inherently less efficacious (29-33); and the increasing prevalence of MRSA will inevitably increase vancomycin use, adding further to the problem of antibiotic-resistant gram-positive bacteria.
Antimicrobial resistance to penicillin, methicillin, or vancomycin is an unavoidable consequence of the selective pressure of antibiotic exposure. Although the details of the epidemiology of staphylococcal drug resistance may change, the fundamental forces driving it are similar. The question is not whether resistance will occur, but how prevalent resistance will become. Minimizing the antibiotic pressure that favors the selection of resistant strains is essential to controlling the emergence of these strains in the hospital and the community, regardless of their origins.
vol7no2/chambers.htm
image-23, mrsa infection on knee,
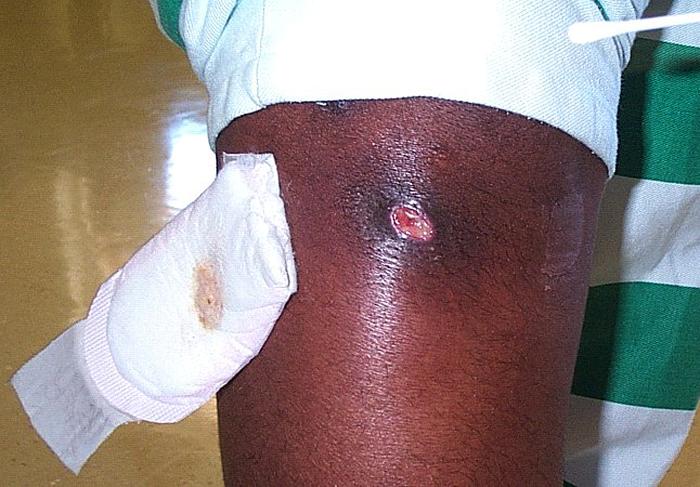
Knee Infection - Click for larger image
Causes
Although the survival tactics of bacteria contribute to antibiotic resistance, humans bear most of the responsibility for the problem. Leading causes of antibiotic resistance include:
Unnecessary antibiotic use in humans. Like other superbugs, MRSA is the result of decades of excessive and unnecessary antibiotic use. For years, antibiotics have been prescribed for colds, flu and other viral infections that don't respond to these drugs, as well as for simple bacterial infections that normally clear on their own.
Antibiotics in food and water. Prescription drugs aren't the only source of antibiotics. In the United States, antibiotics can be found in beef cattle, pigs and chickens. The same antibiotics then find their way into municipal water systems when the runoff from feedlots contaminates streams and groundwater. Routine feeding of antibiotics to animals is banned in the European Union and many other industrialized countries. Antibiotics given in the proper doses to animals who are sick don't appear to produce resistant bacteria.
Germ mutation. Even when antibiotics are used appropriately, they contribute to the rise of drug-resistant bacteria because they don't destroy every germ they target. Bacteria live on an evolutionary fast track, so germs that survive treatment with one antibiotic soon learn to resist others. And because bacteria mutate much more quickly than new drugs can be produced, some germs end up resistant to just about everything. That's why only a handful of drugs are now effective against most forms of staph.
DS00735/DSECTION=3
Statistics
Prevalence of S. aureus and MRSA colonization:
Approximately 32% (89.4 million persons) and 0.8% (2.3 millions persons) of the U.S. population is colonized with S. aureus and MRSA respectively. (Kuehnert MJ et al. Journal of Infectious Diseases. 2006;193:172-9.) Staphylococcal Disease Burden
In Hospitals:
The proportion of healthcare-associated staphylococcal infections that are due to MRSA has been increasing: 2% of S. aureus infections in U.S. intensive-care units were MRSA in 1974, 22% in 1995, and 64% in 2004. (Klevens RM et al. Clinical Infectious Diseases 2006;42:389-91)
There are an estimated 292,000 hospitalizations with a diagnosis of S. aureus infection annually in U.S. hospitals. Of these approximately 126,000 hospitalizations are related to MRSA. (Kuehnert MJ et al. Emerging Infectious Diseases. 2005;11:868-72.)
Skin and Soft Tissue Infections:
Each year from 2001 through 2003 there were an estimated 12 million outpatient (i.e., physician offices, emergency and outpatient departments) healthcare visits for suspected S. aureus skin and soft tissue infections in the United States. (McCaig LF et al. Emerging Infectious Diseases. 2006;12:1715-23.)
In 2004, approximately 76% of purulent (i.e., containing pus) skin and soft tissue infections (SSTIs) in adults seen in 11 emergency departments were caused by S. aureus. Of these infections 78% were caused by MRSA and overall MRSA caused 59% of all SSTIs. (Moran GJ et al. New England Journal of Medicine. 2006; 355:666-74.)
Invasive Infections:
Invasive (i.e., serious) MRSA infections occur in approximately 94,000 persons each year and are associated with approximately 19,000 deaths. Of these infections, about 86% are healthcare-associated and 14% are community-associated. (Klevens et al. Journal of the American Medical Association 2007;298(15):1763-1771
Image-24, mrsa among ICU patients,
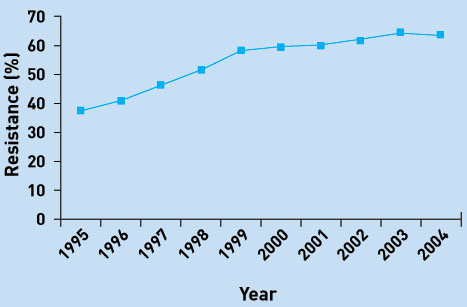
MRSA Among ICU Patients - Click for larger image
Treatment
The good news is that MRSA is treatable. By definition, MRSA is resistant to some antibiotics. But other kinds of antibiotics still work. Bactrim and Vancocin (vancomycin) are often the first drugs used. Other options are Cleocin, Levaquin, Cubicin, Zyvox, and Synercid. Some of these antibiotics may need to be given intravenously. There is also emerging antibiotic resistance being seen with some of these medications as well. Antibiotics aren't always necessary. If you have a skin boil, your doctor may just make an incision and drain it. If you are prescribed antibiotics, follow your health care provider's instructions precisely. Never stop taking your medicine, even if you're feeling better. If you don't take all of your medicine, some of the strongest staph bacteria may survive. These survivors then have the potential to become resistant to the antibiotic. They also could reinfect you or infect someone
understanding-mrsa-detection-treatment
Both hospital and community associated strains of MRSA still respond to certain medications. In hospitals and care facilities, doctors generally rely on the antibiotic vancomycin to treat resistant germs. CA-MRSA may be treated with vancomycin or other antibiotics that have proved effective against particular strains. Although vancomycin saves lives, it may grow resistant as well; some hospitals are already seeing outbreaks of vancomycin-resistant MRSA. To help reduce that threat, doctors may drain an abscess caused by MRSA rather than treat the infection with drugs.
DS00735/DSECTION=7
In Conclusion:
Hospitals are fighting back against MRSA infection by using surveillance systems that track bacterial outbreaks and by investing in products such as antibiotic-coated catheters and gloves that release disinfectants.
Still, the best way to prevent the spread of germs is for health care workers to wash their hands frequently, to properly disinfect hospital surfaces and to take other precautions such as wearing a mask when working with people with weakened immune systems.
In the hospital, people who are infected or colonized with MRSA are placed in isolation to prevent the spread of MRSA to other patients and healthcare workers.Visitors and healthcare workers caring for isolated patients may be required to wear protective garments and must follow strict handwashing procedures.
What you can do in the hospital
Here's what you can do to protect yourself, family members or friends from hospital-acquired infections.
- Ask all hospital staff to wash their hands or use an alcohol-based hand sanitizer before touching you — every time.
- Wash your own hands frequently.
- Make sure that intravenous tubes and catheters are inserted under sterile conditions, for example, the person inserting them wears a mask and sterilizes your skin first.
Image-22, more mrsa awareness poster,

MRSA Awareness Poster - Click for larger image
What you can do in your community
Protecting yourself from MRSA in your community — which might be just about anywhere — may seem daunting, but these common-sense precautions can help reduce your risk:
- Wash your hands. Careful hand washing remains your best defense against germs. Scrub hands briskly for at least 15 seconds, then dry them with a disposable towel and use another towel to turn off the faucet. Carry a small bottle of hand sanitizer containing at least 62 percent alcohol for times when you don't have access to soap and water.
- Keep personal items personal. Avoid sharing personal items such as towels, sheets, razors, clothing and athletic equipment. MRSA spreads on contaminated objects as well as through direct contact.
- Keep wounds covered. Keep cuts and abrasions clean and covered with sterile, dry bandages until they heal. The pus from infected sores may contain MRSA, and keeping wounds covered will help keep the bacteria from spreading.
- Shower after athletic games or practices. Shower immediately after each game or practice. Use soap and water. Don't share towels.
- Sit out athletic games or practices if you have a concerning infection. If you have a wound that's draining or appears infected — for example is red, swollen, warm to the touch or tender — consider sitting out athletic games or practices until the wound has healed.
- Sanitize linens. If you have a cut or sore, wash towels and bed linens in a washing machine set to the "hot" water setting (with added bleach, if possible) and dry them in a hot dryer. Wash gym and athletic clothes after each wearing.
- Get tested. If you have a skin infection that requires treatment, ask your doctor if you should be tested for MRSA. Doctors may prescribe drugs that aren't effective against antibiotic-resistant staph, which delays treatment and creates more resistant germs. Testing specifically for MRSA may get you the specific antibiotic you need to effectively treat your infection.
- Use antibiotics appropriately. When you're prescribed an antibiotic, take all of the doses, even if the infection is getting better. Don't stop until your doctor tells you to stop. Don't share antibiotics with others or save unfinished antibiotics for another time. Inappropriate use of antibiotics, including not taking all of your prescription and overuse, contributes to resistance. If your infection isn't improving after a few days of taking an antibiotic, contact your doctor. ]
DS00735/DSECTION=8